Welcome to Arta’s First Blog!
If you’re reading this, welcome to Arta’s first blog post and thanks for tuning in! This is the first of many that we will provide on the topics of cultured meat science and the power of data to improve novel food production. In particular, we will focus on the application of “Omics” data, across the cellular agriculture R&D pipeline. As this post is the first, we will begin with somewhat of a generalist introduction and the laying out of our stall if you will!
Introduction to Cultured Meat and “Omics”
In the world of sustainable food technology, cultured meat represents a paradigm-shifting advancement in comparison with anything that has come before. It promises a future of eco-friendly and ethical food production, and an end to some of the current unsustainable processes of animal agriculture. It is real meat. From real animals. But NO animals were harmed in the lead-up to it (soon!) landing on your plate. There have been countless reviews and resources published that espouse the process and the potential of cultured meat. As such, we won’t focus on reviewing cultured meat in any great detail, but instead, we would direct you to read Mark Post’s all-encompassing review and the Good Food Institute’s Science of Cultured Meat Tour de Force.
Omic technologies represent similarly groundbreaking advancements for all fields of biological science and have already been responsible for seismic changes in biotechnology, healthcare, agriculture and even criminal justice. While Omics typically refers to a group of tools (Genomics, Proteomics etc.), they have become fully fledged areas of research and science in their own right, with over 30,000 publications citing “Omics” in just 22 years (Figure 1).
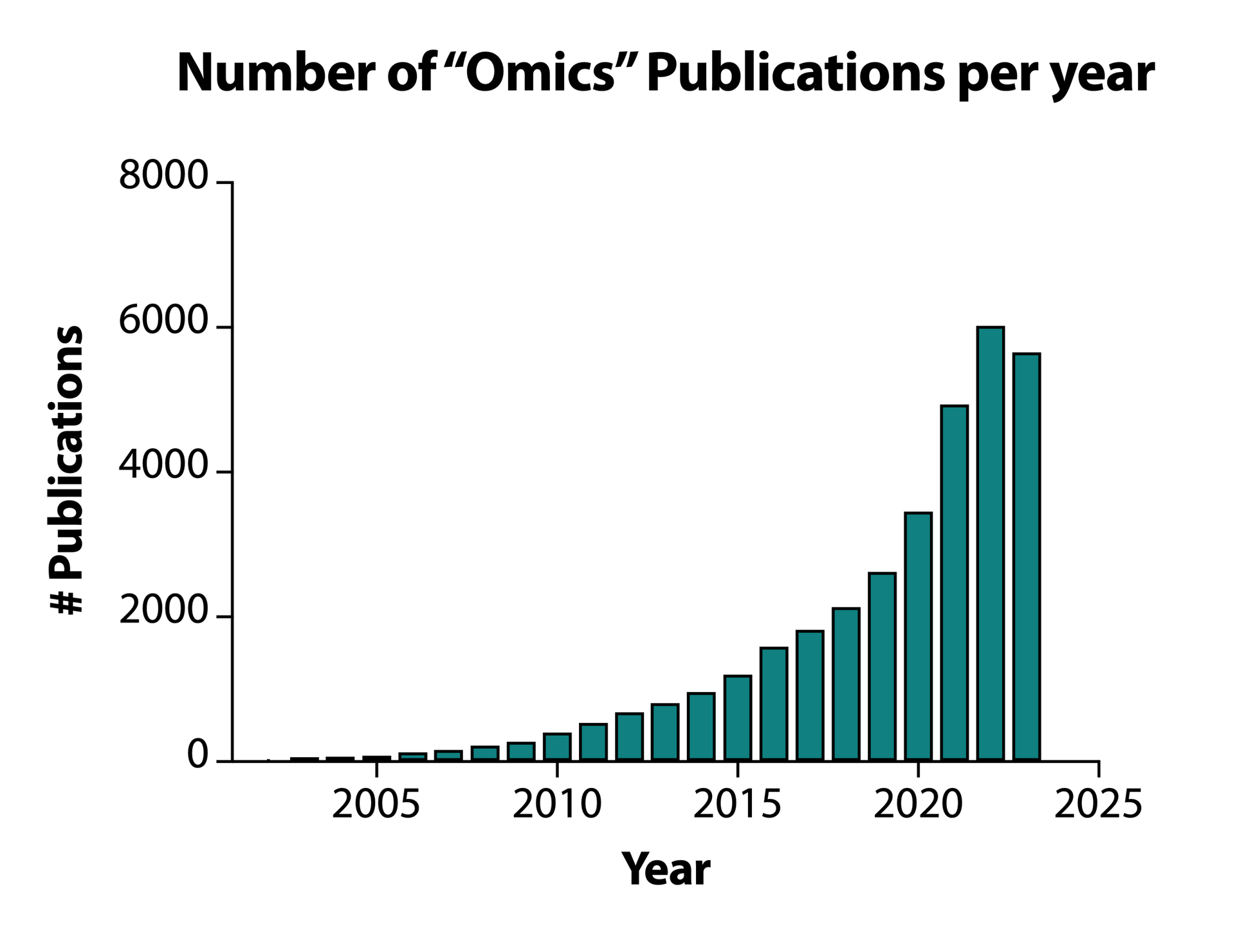
Figure 1. Number of papers citing "Omics" per year since the first use of the term in 2001
Omics technologies now represent a cornerstone of modern biotechnology, agriculture and healthcare, offering an in-depth look at the various molecules and processes that make up a living cell. These tools have revolutionised our understanding of biological systems, allowing us to examine the extraordinary complexity of life with unprecedented molecular detail. They encompass a range of disciplines and tools, with each focusing on a different aspect of the cell:
- Genomics: The study of genomes, providing insights into the complete DNA sequence of organisms - i.e. reading life’s blueprint.
- Transcriptomics: Focusing on RNA (the messages encoded by DNA in coding regions of genomes), it reveals information about patterns of gene expression.
- Proteomics: Examining the entire set of proteins produced (translated from the RNA messages), which is crucial for understanding cellular functions, as function ultimately happens at the level of the proteome.
- Metabolomics: Analyzing the complete set of metabolites, which are key indicators of cellular processes - these indicators come largely from the functioning of proteins, like enzymes.
- Multiomics and Systems Biology: The integration and combination of these omic technologies, known collectively as multiomics, allows for a comprehensive view of biological systems (i.e. Systems Biology!). This holistic approach is essential in systems biology, offering us a considerably more detailed window on biology than we have ever had before.
Together, these technologies enable us to tease apart the intricacies of cellular and organism functions and open up our window onto biology.
In the still relatively nascent field of cultured meat, omics technologies present us with a world of opportunity when it comes to the scientific and manufacturing challenges involved, as well as the regulatory hurdles that still face us in bringing cultured meat to market and into the mainstream.
Why should cultured meat research and development leverage Omics tools?
It’s easy to get caught up in applying new and shiny technologies to biological research fields, often where they’re not really needed. It’s also equally easy to quickly get lost in the vast swathes of data that these technologies provide you and not yield any meaningful insight. Not falling into these traps in any industry is critical to the success of your product R&D. But, avoiding this enticing allure of big data in an industry that must remain so laser-focussed on bringing products to market, in this current climate, is of the utmost importance!
Now, this may sound at odds to our entire offering at Arta - Omics and data for cellular agriculture - in fact, it’s quite the opposite. The power of these technologies to transform your product development and scientific R&D processes, means that now is exactly the right time to leverage this complex, technical Omics toolkit. Leveraging these tools can help give your team the competitive advantage to stand out above the rest in this harsh winter we’re currently seeing in Cell Ag, as well as overcome some of the major challenges that we face in the industry. Let’s delve into what these challenges are, and why Omics can help.
Firstly, much of the cellular agriculture industry is focussed on solving really, really hard problems, many of which have never been solved before (at least not in this breadth and depth). Yes, there are pieces of the puzzle that have been addressed by the Biopharma industry. And there are learnings that can be applied from brewing, or protein production, or industrial scale food manufacture. But there are also numerous technological tools and approaches needed for Cell Ag that are only just finding the light of day outside research labs, like Crispr base-editing or in-line metabolite monitoring, to name just a few. There is also a significant need for technologies that don’t even exist yet! Which is why many companies in this space take a unique and proprietary approach to their product development, and are funded largely on the basis of intellectual property. For the generation of new technologies for really big problem-solving, big Omics data can help by offering truly novel and broad insights into your cellular systems, which can help deliver on some of the hard problems we are all trying to solve.
Next, we are dealing with biological systems that have almost never been investigated, in comparison with “Model Organism” species, like mice and rats. On average, and wholly unsurprisingly, in the past 70 years there have been 10 times more publications per year using mice (murine), than there have cows or pigs (bovine and porcine, respectively) (Figure 2). This puts the cultured meat industry on the immediate back foot technically, as there simply is not the same depth of understanding of these organisms as there is of “simpler” mammals, like mice and rats. The technical debt that we face with the use of non-model organisms means that there are fewer protocols to isolate and select cell-lines from these animals, fewer publications identifying key metabolic differences between mice and cows, and fewer media formulations tailored to agricultural animal cell culture. When considering species beyond cows, pigs and chickens, this knowledge chasm becomes even greater!
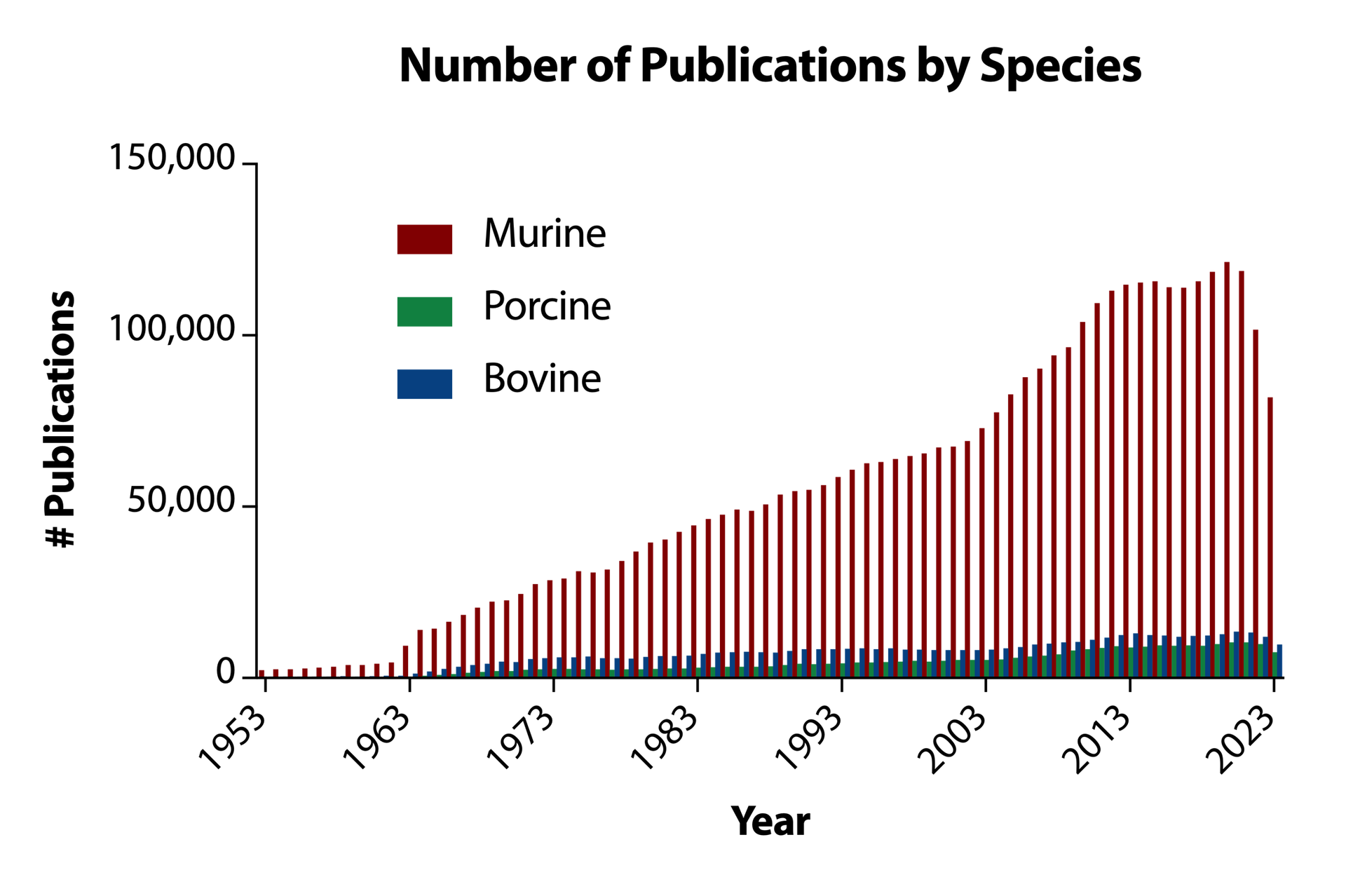
Figure 2. Number of papers citing the words mouse (murine), cow (bovine) and pig (porcine) in the past 70 years
Luckily, we’re not re-writing the book entirely, and we don’t need to publish 10x more on cow cell culture in the next 2 years for cultured meat science to succeed, as there are many, many extrapolations that can be made from our understanding of mouse and human biology. However, the must still industry try to make up for some of this knowledge gap, by developing its own technologies, improving its biological understanding of these newer species and managing to make delicious products, all at the same time. We are building the bike, riding the bike and repairing the bike, all at the same time!
These challenges give us a huge opportunity to leverage contemporary research tools like multiomics, as they can significantly speed up our rate of understanding of these lesser-understood organisms with unparalleled breadth and depth. For the first time in history, we can start to generate insights on new species and cell functions faster than ever before. In a single multiomics experiment, capturing metabolism, gene expression and protein-level data, you can truly begin to understand a cell’s function and start to overcome the knowledge gap that comes with working with cells from cows, or chickens, or zebra, or glacier toothfish!
Thanks for reading our first blog!
If you liked this one, feel free to get in touch with me at alex@artabioanalytics.com, and we can continue the discussion! And stay tuned for follow-ups, including next in stall “How to use omics technologies in cultured meat production”.
Comments